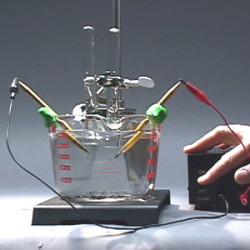
In chemistry and manufacturing, electrolysisis a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially highly important as a stage in the separation ofelements from naturally occurring sources such as ores using an electrolytic cell. Industrial uses Hall-Heroult process for producing aluminium Productionkof aluminium, lithium, sodium,potassium, magnesium, calcium Coulometric techniques can be used to determine the amount of matter transformed during […]
 In chemistry and manufacturing, electrolysisis a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction.
In chemistry and manufacturing, electrolysisis a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction.
Electrolysis is commercially highly important as a stage in the separation ofelements from naturally occurring sources such as ores using an electrolytic cell.
Industrial uses
- Productionkof aluminium, lithium, sodium,potassium, magnesium, calcium
- Coulometric techniques can be used to determine the amount of matter transformed during electrolysis by measuring the amount of electricity required to perform the electrolysis.
- Production of chlorine and sodium hydroxide.
- Production of sodium chlorate andpotassium chlorate.
- Production of perfluorinated organic compounds such as trifluoroacetic acid.
- Production of electrolytic copper as acathode, from refined copper of lower purity as an anode.
Electrolysis has many other uses:
- Electrometallurgy is the process of reduction of metals from metallic compounds to obtain the pure form of metal using electrolysis. For example, sodium hydroxide in its molten form is separated by electrolysis into sodium and oxygen, both of which have important chemical uses. (Water is produced at the same time.)
- Anodization is an electrolytic process that makes the surface of metals resistant tocorrosion. For example, ships are saved from being corroded by oxygen in the water by this process. The process is also used to decorate surfaces.
- A battery works by the reverse process to electrolysis.
- Production of oxygen for spacecraft and nuclear submarines.
- Electroplating is used in layering metals to fortify them. Electroplating is used in many industries for functional or decorative purposes, as in vehicle bodies and nickel coins.
- Production of hydrogen for fuel, using a cheap source of electrical energy.
- Electrolytic Etching of metal surfaces like tools or knives with a permanent mark or logo.
Electrolysis is also used in the cleaning and preservation of old artifacts. Because the process separates the non-metallic particles from the metallic ones, it is very useful for cleaning old coins and even larger objects.